VICTORIA Study (Vericiguat in Patients with Heart Failure with Reduced Ejection Fraction)
“Verquvo is indicated for the treatment of symptomatic chronic heart failure in adult patients with reduced ejection fraction who are stabilised after a recent decompensation event requiring IV therapy”
- Mortality & morbidity very high in HFrEF patients who require hospitalisation/IV diuretics
- sGC-mediated production of cGMP essential for normal CV function
- In HF, reduced NO bioavailability results in relative sGC deficiency & reduction in cGMP
- Verquvo - oral, soluble, NO independent, direct sGC stimulator
- VICTORIA study designed to assess efficacy & tolerability in HFrEF patients
- Patients had undergone recent hospitalisation/received IV diuretics
HFrEF, heart failure with reduced ejection fraction; IV, intravenous; sGC, soluble guanylate synthase; cGMP, cyclic guanosine monophosphate; CV, cardiovascular; HF, heart failure; NO, nitric oxide
1. Armstrong PW et al. JACC Heart Fail 2018; 6(2): 96-1042; 2. ArmstrongPW et al. N Engl J Med. 2020;382:1883–1893

*If the 10 mg target dose was not reached, then up-titration was considered at subsequent study visits, based on protocol-specified criteria.
AF, atrial fibrillation; BNP, brain natriuretic peptide; NT-proBNP, N-terminal pro B-type natriuretic peptide; eGFR, estimated glomerular filtration rate; HFH, heart failure hospitalisation; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; od, once daily; Q16W, every 16 weeks; SR, sinus rhythm;
1. Adapted from Armstrong PW et al. JACC Heart Fail. 2018;6:96–104;
2. Adapted from Armstrong PW et al. N Engl J Med. 2020;382:1883–1893

*Intention to treat (ITT) population, ‡Calculations: annual NNT = 100/4.2 = 24
-
Median follow-up duration for primary endpoint: 10.8 months
-
Event rates for Verquvo and placebo per 100 patient-years were 33.6 and 37.8, respectively
CV, cardiovascular; HFH, heart failure hospitalisation; HR, hazard ratio; CI, confidence interval; ARR, absolute risk reduction; NNT, numbers needed to treat
Adapted from Armstrong PW et al. N Engl J Med. 2020;382:1883–1893

*For patients with multiple events, only the first event contributing to the composite endpoint is counted in the table.
#HR (Verquvo versus placebo) and CI calculated from Cox proportional-hazards model controlling for stratification factors (defined by region and race); ‡Calculated from stratified log-rank test with stratification factors defined by region and race; ¶deaths included in the primary and secondary composite outcomes were not preceded by a hospitalisation for HF. Based on data up to the primary completion date (18 June 2019)
Adapted from Armstrong PW et al. N Engl J Med. 2020;382:1883–1893
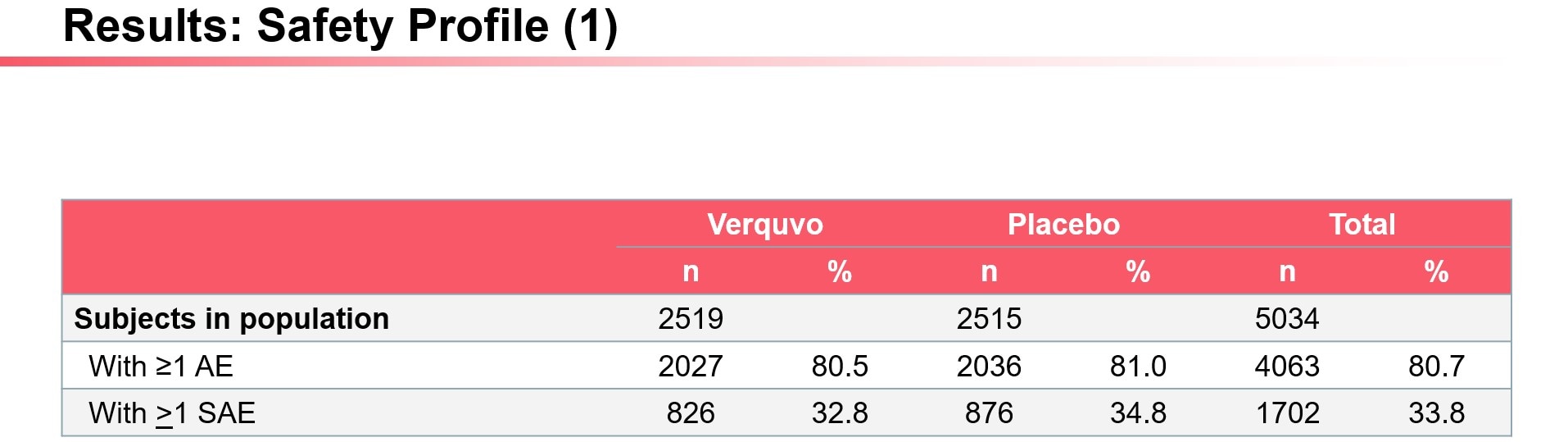
AE, adverse event; SAE, serious adverse event
Armstrong PW et al. N Engl J Med. 2020;382:1883–1893 Supplementary Appendix

*Based on Miettinen & Nurminen method. Note: Includes events/measurements from the day of first dose of study drug to 14 days after the last dose of study drug. Based on data up to the primary analysis cut-off date (18 Jun 2019)
CI, confidence interval
Armstrong PW et al. N Engl J Med. 2020;382:1883–1893 Supplementary Appendix

SBP, systolic blood pressure; BP, blood pressure; SD standard deviation
Armstrong PW et al. N Engl J Med. 2020;382:1883–1893 Supplementary Appendix

GI, gastrointestinal
Armstrong PW et al. N Engl J Med. 2020;382:1883–1893 Supplementary Appendix
Verquvo Data further information Slide 1 References:
- Armstrong PW et al. JACC Heart Fail 2018; 6(2): 96-104 Armstrong PW et al. JACC Heart Fail 2018; 6(2): 96-104 Return to content
Verquvo Data further information Slide 2 References:
- Armstrong PW et al. JACC Heart Fail 2018; 6(2): 96-104 Armstrong PW et al. JACC Heart Fail 2018; 6(2): 96-104 Return to content
- Armstrong PW et al. N Engl J Med. 2020;382:1883–1893 Armstrong PW et al. N Engl J Med. 2020;382:1883–1893 Return to content
Verquvo Data further information Slide 3 References:
- Armstrong PW et al. JACC Heart Fail 2018; 6(2): 96-104 Armstrong PW et al. JACC Heart Fail 2018; 6(2): 96-104 Return to content
- Armstrong PW et al. N Engl J Med. 2020;382:1883–1893 Armstrong PW et al. N Engl J Med. 2020;382:1883–1893 Return to content
Verquvo Data further information Slide 4 References:
- Armstrong PW et al. JACC Heart Fail 2018; 6(2): 96-104 Armstrong PW et al. JACC Heart Fail 2018; 6(2): 96-104 Return to content
Verquvo Data further information Slide 5 References:
- Armstrong PW et al. JACC Heart Fail 2018; 6(2): 96-104 Armstrong PW et al. JACC Heart Fail 2018; 6(2): 96-104 Return to content
- Armstrong PW et al. N Engl J Med. 2020;382:1883–1893 Armstrong PW et al. N Engl J Med. 2020;382:1883–1893 Return to content
Verquvo Data further information Slides 6 - 17 References:
- Armstrong PW et al. N Engl J Med. 2020;382:1883–1893 Armstrong PW et al. N Engl J Med. 2020;382:1883–1893 Return to content
Verquvo Data further information Slide 19 - 22 References:
- Armstrong PW et al. N Engl J Med. 2020;382:1883–1893 Supplementary Appendix Armstrong PW et al. N Engl J Med. 2020;382:1883–1893 Supplementary Appendix Return to content
Verquvo Data further information Slide 23 References:
- Ezekowitz JA et al. JACC. 2020;8:931–939 Ezekowitz JA et al. JACC. 2020;8:931–939 Return to content
Verquvo Data further information Slide 24 References:
- Armstrong PW et al. N Engl J Med. 2020;382:1883–1893 Armstrong PW et al. N Engl J Med. 2020;382:1883–1893 Return to content
- Ezekowitz JA et al. JACC. 2020;8:931–939 Ezekowitz JA et al. JACC. 2020;8:931–939 Return to content
Verquvo Data further information Slide 25-27 References:
- Ezekowitz JA et al. JACC. 2020;8:931–939 Ezekowitz JA et al. JACC. 2020;8:931–939 Return to content
Verquvo Data further information Slide 28 References:
- Ezekowitz JA et al. JACC. 2020;8:931–939 (Supplementary Appendix) Ezekowitz JA et al. JACC. 2020;8:931–939 (Supplementary Appendix) Return to content
Verquvo Data further information Slide 29-31 References:
- Ezekowitz JA et al. JACC. 2020;8:931–939 Ezekowitz JA et al. JACC. 2020;8:931–939 Return to content
PP-VER-GB-0029 | November 2022


